
TRD Consulting - Innovative Toxicology
Regulatory Toxicology
Expert Regulatory Toxicology Services for Pharmaceutical, Biotech and Chemical Industries

TRD Consulting - Innovative Toxicology
Study Designing and Monitoring of Nonclinical Safety and Pharmacology Studies (in vitro and in vivo)
Design & Monitoring of In Vitro and In Vivo Toxicology Studies by Dr. Ramesh Subramani

TRD Consulting - Innovative Toxicology
Toxicology Risk Assessment Consulting
PDE, OEL, QSAR, E&L, Carcinogenicity and Genotoxic Impurity Evaluation Services by Dr. Ramesh Subramani

TRD Consulting - Innovative Toxicology
GLP Implementation and Compliance Advisory
Good Laboratory Practice (GLP) Consulting by Dr. Ramesh Subramani

TRD Consulting - Innovative Toxicology
Strategic IND/NDA/ANDA/BLA Support for Global Regulatory Submissions
End-to-End Regulatory Toxicology Support by Dr. Ramesh Subramani
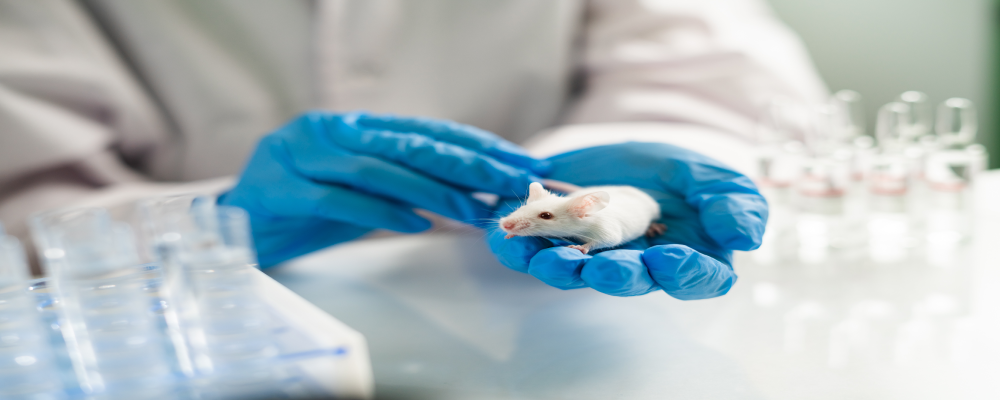
TRD Consulting - Innovative Toxicology
Animal Welfare and Ethical Study Design Guidance
Humane, Regulatory-Compliant Preclinical Study Design by Dr. Ramesh Subramani

















